 |
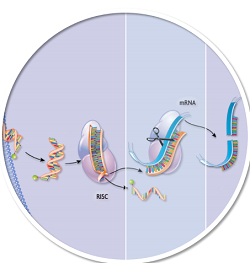
| An illustration of the RNAi pathway by which targeted genes are silenced using small interfering RNA. The picture features the RNA-induced silencing complex and messenger RNA, which are critical components of the naturally occurring process.--Courtesy of Alnylam |
Saying it is on track to exceed its goal of having 5 key products in clinical development through 2015, Alnylam ($ALNY) recently announced that it intends to focus on three strategic therapeutic areas, or what it dubs "STArs," as it races to commercialize its first candidate delivered using the RNAi pathway and reassure sometimes antsy investors.
The STArs are genetic medicine, with 8 announced candidates, cardiometabolic disease (four candidates) and hepatic infectious disease (three candidates). They will share some commonalities, such as a continued focus on the liver, where RNAi delivery has shown the most promise due to favorable biology. The candidates will be administered subcutaneously or intravenously, with monthly or quarterly dosing a possibility for those focused on cardiometabolic disease and hepatic infectious disease, according to company slides.
In the genetic medicine STAr, the two candidates for TTR-mediated amyloidosis, patisiran and revusiran, are in Phase III. The furthest-along candidate in the cardiometabolic STAr is ALN-PCSsc, focused on hypercholesterolemia, and its preclinical hepatitis B candidate (ALN-HBV) is the only hepatic infectious disease candidate to leave the discovery stage, company slides show.
"Based on a wealth of growing preclinical and clinical data, we now believe that the breadth of Alnylam's opportunities for RNAi therapeutics extends beyond the original framework of our 'Alnylam 5x15' strategy. Accordingly, we are excited to announce an evolution in our growth strategy with a focus on development and commercialization of RNAi therapeutics in three Strategic Therapeutic Areas," said Alnylam CEO John Maraganore in a statement.
More details are provided in an almost four-hour R&D day company presentation featuring multiple speakers and various Q&A sessions. Constant communication is important for a company like Alnylam, whose focus on the nascent RNAi delivery mechanism means it is subject to the whims of investors.
After its stock tumbled in April following news that Novartis ($NVS) was slamming the brakes on its RNAi research, Alnylam COO Barry Greene flipped the script, telling FierceDrugDelivery that Big Pharma has "never been able to innovate."
Announcements like this one are designed to provide investors with much-needed reassurance that everything is on track at the company. The biggest scientific and commercialization barrier is, you guessed it, drug delivery.
"When we say delivery, we're talking about getting the siRNA [small interfering RNA], which is the drug that intermediates RNAi into the cytoplasm of the cell, which is where the RNAi machinery works," Greene said. Alnylam's GalNAc-siRNA platform uses the sugar molecule GalNAc conjugated to the RNA molecule to enable subcutaneous delivery.
- read the release
- here are the company slides
- a link to the R&D day presentation (reg. req.)