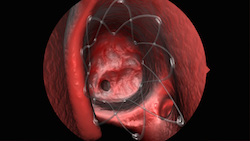
 |
| Propel's springlike design conforms to patient's unique anatomy and props open the sinus--Courtesy of Intersect ENT |
Fierce 15 member Intersect ENT said a study of its bioabsorbable steroid-releasing sinus implant met its primary endpoint, as the company seeks to expand the indication of its drug delivery tech to include placement in the frontal sinuses, located behind the eyebrows.
Compared with surgery alone, the Propel mini device demonstrated statistically significant 38% relative reduction in the need for postoperative interventions like additional surgical procedures or oral steroid prescription, the company said in a release.
Propel mini is currently approved to be placed in the ethmoid sinuses, which are located right behind the bridge of the nose, and accounts for 25% of sinus surgeries.
"Frontal sinus disease contributes greatly to the debilitating symptoms of chronic sinusitis, including severe headaches, and is known to be the most difficult sinus to manage," said the study's principal investigator, Dr. Tim Smith of Oregon Health & Science University. "These results offer an exciting prospect for this group of patients."
The results were only preliminary. According to a company presentation, Intersect is enrolling 80 more patients in the study. They will receive its investigational Nova implant, which can be installed via an in-office procedure. ClinicalTrials.gov says that each patient will receive one implant, and the untreated side of the face serves as the control.
CEO Lisa Earnhardt said the company's next step will be compiling and submitting the results to the FDA this year, in a statement. She has previously said that the device improves upon both intranasal steroids, which only deliver about 2% of the drug to the nasal cavity, and oral steroids, which come with a host of side effects despite their potency.
The company's Propel and Propel mini are the first and only FDA-approved steroid release sinus implants. They release mometasone furoate into the sinus lining before dissolving and being absorbed by the body over a period of 30-45 days. The implants are designed to maintain the open passages created in surgery, Intersect says.
New implants are in the pipeline.
In July, the ear, nose and throat device specialist said it would start a pivotal trial for bioabsorbable, steroid-releasing sinus implant dubbed Nova, which is designed to be used in the smaller frontal and maxillary sinus openings.
In the first half the year, the company reported revenues of $28.6 million and posted a net loss of $11.1 million.
- read the release
Special Reports: Top women in medical devices - Lisa Earnhardt, Intersect ENT | FierceMedicalDevices' 2013 Fierce 15 - Intersect ENT