 |
|
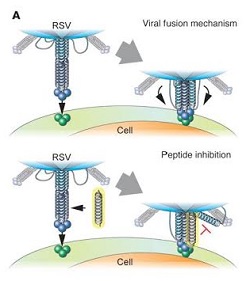
In this illustration of the mechanism of the therapy, the yellow helix is a decoy that prevents proper fusion of the RSV with the host cell.--Courtesy of Shyam Mohapatra
|
Researchers in Boston and Tampa, FL, have developed a unique delivery mechanism that inhibits respiratory syncytial virus (RSV) using double-stapled peptides and nanoparticles. They believe their research has implications for the treatment of other viruses as well.
RSV causes severe respiratory tract viral illnesses such as pneumonia and bronchiolitis, and is responsible for the deaths of 400 infants and 10,000 elderly in the U.S. every year, according to the team's paper published in The Journal of Clinical Investigation on April 17.
The virus causes more than 160,000 deaths across the globe annually, Shyam Mohapatra, a senior author of the study and director of the University of South Florida Nanomedicine Research Center, said in an interview. He added that current treatments for the virus are inadequate or overly expensive.
The peptides disrupt the fusion process that the virus uses to bind to and infect the epithelial cells that line the nose and lungs. Mice given nose drops containing the peptides had lower levels of RSV in the nose and lungs than the control group, the researchers said.
A key innovation was the employment of a technique known as hydrocarbon stapling to create "double-stapled" RSV peptides. The technique involves bioengineering the peptides by inserting synthetic amino acids and other molecules within their helix structure so that they retain their natural shape and are not degraded by the body's enzymes.
"Each peptide has a helical structure and you need to make sure that it stays helical so that each interaction is perfect. Once the peptide has lost its helical structure, the domain interaction will not work," Mohapatra said.
In addition, the team found that the therapy worked better when the peptides were encapsulated in nanoparticles and delivered to the lungs via the trachea. Mohapatra said the nanoparticles "provide a second layer of protection" and extend the half-life of the peptides to 72 hours, which could reduce the number of doses required.
Mohapatra said the researchers are discussing the possibility of testing the therapy in human clinical trials with Cambridge, MA-based Aileron Therapeutics. Dr. Loren Walensky of Harvard Medical School, a coauthor of the paper, is one of the company's advisers.
In addition, Tampa, FL-based TransGenex Nanobiotech, co-founded by Mohapatra, could help further develop the nanoparticle technology, the scientist said.
The breakthrough could have implications for the treatment of other viruses as well, Mohapatra added. Therapeutic peptides that inhibit viral fusion have been used to combat HIV-1 infection, but their success was limited due to the peptides' lack of structural stability and tendency to degrade in the body. The researchers suggest peptide double-stapling could address those issues.
"New technologies that fortify lengthy peptides, such as hydrocarbon double stapling, are reinvigorating efforts to optimize natural peptides for therapeutic application," the researchers wrote in the The Journal of Clinical Investigation.
- here's the release
- and the research article