 |
| Courtesy of BioDelivery Sciences |
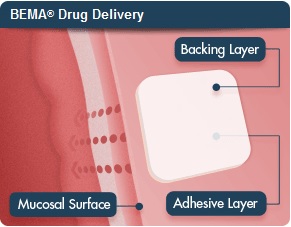
Endo Pharmaceuticals ($ENDP) and BioDelivery Sciences International ($BDSI) announced positive results from two Phase III studies of their Belbuca buccal film to manage severe chronic pain by quickly delivering buprenorphine via the inside of the cheek. The candidate uses BDSI's BioErodible MucoAdhesive delivery platform.
One study involved pain sufferers who had previously received opioid therapy, while the other involved "opioid-naive" patients. All 971 patients received either the candidate, which delivers the drug buprenorphine, or a placebo buccal film.
Almost two-thirds of patients in each treatment arm experienced a reduction in pain of greater than 30%. The placebo patients didn't do as well: 47% of those in the opioid-naive study and 31% of patients who had previously received opioid therapy had pain reduction of greater than 30%.
"In these studies, the investigational study drug Bema buprenorphine demonstrated a consistent, statistically significant improvement in patient-reported pain relief," said lead investigator Dr. Joseph Gimbel of the Arizona Research Center in Phoenix in a statement. "In both trials, Bema buprenorphine was effective in reducing pain at every week studied, and in the opioid-experienced trial, the responder analysis was more than twice the rate of placebo. Additionally, the adverse event (AE) profiles were encouraging--the percentage of patients reporting any AE was similar between patients treated with Bema buprenorphine or placebo. I am excited at the potential this new product will bring to the medical community if approved."
The most common adverse events among patients receiving the candidate were nausea (7.5%), vomiting (5.5%) and drug withdrawal syndrome (3.5%).
In February, Endo Pharmaceutical's investigational Belbuca was accepted for review by the FDA, marking a win for BEMA technology to deliver drugs across mucosal surfaces like the inside of the cheek. BEMA platform developer BioDelivery Sciences received a $10 million milestone payment.
The companies hope that the most recent data put them on track for approval. A decision is due by the FDA's PDUFA deadline in October.
- read the release