 |
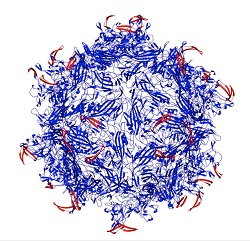
| A virus capsid (blue) with peptides (red) that lock the virus until they are "opened" by enzymes at a tumor site--Courtesy of Rice University |
Researchers at Rice University in Texas have developed a new kind of drug delivery particle: a "tunable" virus that releases a treatment only in the presence of not one but two different enzymes that show elevated levels at the site of a tumor.
In a study published this week in the American Chemical Society journal ACS Nano, researchers designed an adeno-associated virus capsid that unlocks when it encounters the two proteases, binds to the cancer cell and releases either a cell-killing therapy or genes to treat other diseases. The double lock ensures that the treatment is released at the right spot--like a safe deposit box, having two different keys keeps the virus from unloading in an undesired location that has only one of the enzymes present.
"If we were just looking for one protease, it might be at the cancer site, but it could also be somewhere else in your body where you have inflammation," lead author Junghae Suh said in a statement. "This could lead to undesirable side effects. By requiring two different proteases--let's say protease A and protease B--to open the locked virus, we may achieve higher delivery specificity since the chance of having both proteases elevated at a site becomes smaller."
Along with cancer, these activated viruses could also help treat neurological diseases such as stroke, Parkinson's and Alzheimer's, as well as cardiovascular conditions such as heart disease, myocardial infarction and congestive heart failure, according to a report from the university.
And the goal is to create viruses with even more specificity--and more locks.
"To increase the specificity of virus unlocking, you can imagine creating viruses that require many more keys to open," Suh said. "For example, you may need both proteases A and B as well as a cellular receptor to unlock the virus. The work reported here is a good first step toward this goal."
- here's the Rice University report
- and here's the ACS Nano abstract