 |
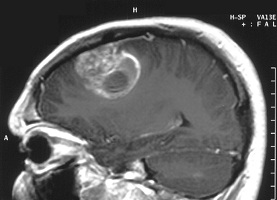
| MRI image of a glioblastoma tumor--Courtesy of NIH |
Not only is Hampton, NJ-based Celldex Therapeutics ($CLDX) operating in the challenging cancer vaccines field, but it's working on a treatment for one of the most difficult-to-treat cancers. But with positive new Phase II survival data in hand, the company could eventually have a winner.
Over the weekend at the American Society of Clinical Oncology (ASCO) annual meeting, the biotech unveiled Phase II study results showing a clear survival benefit for its candidate, Rintega. After 12 months, 45% of patients who received Rintega plus Roche's ($RHHBY) Avastin were alive, compared with 31% of Avastin-only patients. Rintega also notched a median overall survival rate of 11.6 months, besting the control arm's 9.3 months.
With the results in hand, Celldex is conducting ongoing discussions with regulators, it said in a statement. In February, the FDA gave Rintega its breakthrough therapy designation, designed to allow for seamless communication as the body considers the case for an accelerated approval. At the time, Celldex CEO Anthony Marucci noted that the patients have "extremely limited treatment options, with only three new drugs approved in more than 20 years."
Rintega is aimed at treating glioblastoma patients with the EGFRvIII mutation, who typically have a worse prognosis than the overall glioblastoma population. The candidate hasn't always been a star, though, and Pfizer ($PFE) dropped a collaboration with Celldex 5 years ago. Peak sales estimates range from a few hundred million dollars per year to more than $1 billion annually.
With Phase III data coming later this year, Celldex is hoping to avoid the troubles that have faced the cancer vaccine field, with companies such as GlaxoSmithKline ($GSK) and Merck KGaA recently announcing late-stage failures. Northwest Biotherapeutics ($NWBO), with a glioblastoma candidate of its own, has trumpeted individual success stories from a Phase I/II of its DCVax-L, but it has had to fend off critics who have said the trial's size and "informal nature" should prohibit the data from being considered statistically significant.
- here's the release
- get more from FierceBiotech