 BioDelivery Sciences International ($BDSI) has secured a patent for its dissolvable opioid-dependence treatment, and the company is looking to file an application for BEMA Buprenorphine next year. The patent applies to the drug's transmucosal delivery platform, a small strip of film placed on the inside of the cheek that dissolves and administers the anti-opioid buprenorphine.
BioDelivery Sciences International ($BDSI) has secured a patent for its dissolvable opioid-dependence treatment, and the company is looking to file an application for BEMA Buprenorphine next year. The patent applies to the drug's transmucosal delivery platform, a small strip of film placed on the inside of the cheek that dissolves and administers the anti-opioid buprenorphine.
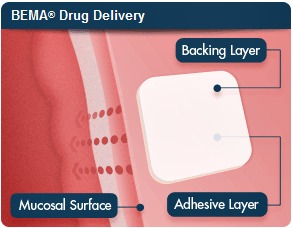
BEMA, which stands for BioErodible MucoAdhesive, adheres to the cheek and dissolves after 15 to 30 minutes, delivering a combination of buprenorphine to quell opioid cravings and naloxone, an opioid antagonist, to prevent abuse.
The API is preferable to methadone for treating opioid addiction, BDSI has said, but BEMA wouldn't be the first treatment to use it. Reckitt Benckiser's Suboxone is currently the only oral buprenorphine treatment on the market, but BDSI believes BEMA's drug delivery tech will allow it to carve out a share of the market.
As BDSI Executive VP Andrew Finn told MedCity News, Suboxone is an under-the-tongue treatment, leading to increased saliva production and the risk of patients swallowing much of the drug before it can be absorbed. BEMA avoids that issue, as its film is coated with a second layer that prevents the buprenorphine from mixing with saliva. Furthermore, patients have reported an unpleasant taste with Suboxone, and BDSI says BEMA doesn't have that problem.
The patent news triggered a milestone payment of $15 million under BDSI's licensing and development agreement with Endo Pharmaceuticals ($ENDP). BDSI sees a big opportunity for BEMA, with CEO Mark Sirgo telling investors that it could reach $300 million in sales if approved, MedCity News reported. The company has already reaped some benefit from the licensing agreement, as its stock price jumped after the deal's January announcement.
(Image courtesy of BDSI)
- here's the company release
- read the MedCity News report