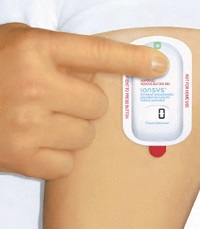
 |
| Ionsys transdermal system--Courtesy of Medicines Company |
New Jersey's The Medicines Co. ($MDCO) has tied the knot with Tokyo's SymBio, a deal that gives the Japanese company the exclusive license to develop and commercialize The Medicine Co.'s Ionsys transdermal pain system in its home country.
Ionsys is a fentanyl iontophoretic transdermal system for acute postoperative pain. The FDA approved the treatment in April, followed by the European Medicines Agency in September. SymBio plans to pay $10 million up front with net sales royalties and regulatory and commercial milestones, the companies said in a release.
It's also the first of The Medicines Co.'s products to reach the Japanese market, the company's president Glenn Sblendorio said in a statement.
 |
| SymBio CEO Fuminori Yoshida |
The Ionsys system is the first needle-free, preprogrammed fentanyl delivery system designed to be controlled by the patient. The patch forgoes normal intravenous analgesia for a transdermal delivery dispensed at the touch of a button via an electrical current.
"We are very excited to add IONSYS to our product portfolio. This product has the potential to change the management of pain in the hospital," SymBio CEO Fuminori Yoshida said. "We hope to take full advantage of an accelerated path to regulatory approval given that IONSYS has already completed a Phase I study in Japanese patients."
- here's the release