 |
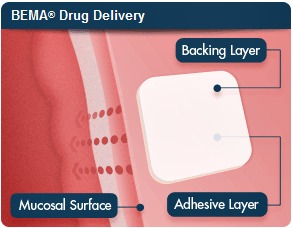
| BDSI's BEMA film adminsters treatment by adhering to the inside of the cheek and dissolving after 15 to 20 minutes--courtesy of BDSI |
BioDelivery Sciences International ($BDSI) is in line for a $2.5 million milestone payment from Meda, as the cancer pain drug Breakyl got its first regulatory clearance and pricing approval in the EU.
Breakyl, which will be marketed by Meda, is formulated to treat flare-ups of cancer pain, and it uses BDSI's patented transmucosal film to deliver quick-acting fentanyl into patients. The platform, called BEMA, is a film that adheres to the inside of the cheek and dissolves 15 to 20 minutes after releasing its API.
BDSI is in line for another $2.5 million from Meda once Breakyl hits the market in the EU, which the company expects to happen later this year.
This latest flush of cash comes on the heels of BDSI's $15 million milestone payment for BEMA Buprenorphine, an opioid-dependence treatment that uses the same inside-the-cheek platform. That drug got its patent in April, and BDSI and partner Endo Pharmaceuticals ($ENDP) plan to file for FDA approval next year.
That Endo deal, announced in January, led to a spike in BDSI's stock, and the company is looking to piggy-back off the high-profile agreement and expand its proprietary film to cash in on new formulations of existing therapeutics, BDSI said in a statement.
- read the company's release