 |
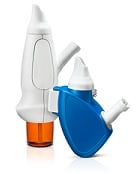
| OptiNose's powder delivery devices with nosepiece and mouthpiece--Courtesy of OptiNose |
Avanir Pharmaceuticals ($AVNR) is set to pay up to $110 million for an experimental inhaled, dry-powder migraine treatment from PA-based devicemaker OptiNose.
OptiNose will collect $20 million up front and another $90 million in milestone and sales target payments upon approval of the AVP-825 device, which makes use of a novel nasal delivery system invented by Norwegian ear, nose and throat specialist and OptiNose founder Per Djupesland. The "bi-directional nasal technology" uses the patient's natural breathing flow for nasal delivery, with both a mouthpiece and a nosepiece. The patient exhales into the mouthpiece to propel drugs into the nasal cavity.
Avanir will take control of the regulatory, manufacturing, supply chain and commercialization of the device, according to a release, and the mid-sized biotech will prepare an NDA to file with the FDA by early 2014.
"Avanir is an ideal partner given its proven track record of successfully developing and commercializing neuroscience products," said OptiNose CEO Peter Miller. "The results from our Phase III clinical study were extremely encouraging and we believe we have a potential treatment that provides pain relief quickly and with few adverse events."
OptiNose completed its Phase III trial in November 2012. The study showed that 68% of patients experienced headache relief after two hours, and as quickly as 15 minutes after treatment.
- here's the release
- and here are videos of the tech