 |
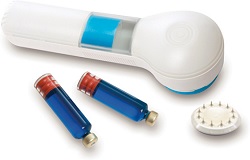
| 3M's Hollow Microstructure Transdermal System--Courtesy of 3M |
3M announced last week that its Hollow Microstructure Transdermal System for home drug delivery by patients is available for licensing at the Drug Delivery Partnerships conference in Boca Raton, FL.
The device offers intradermal delivery of biologic formulations ranging from 0.5 to 2 mL. Other product features include a protective safety cap for the 12-microneedle array and a status indicator window to show patient's the administration's progression, according to the product website.
Delivery takes 15 seconds to 15 minutes depending on the drug's volume and formulation, 3M says.
"From the foundation laid by our recent human study, we are excited to extend our hollow microneedle device and expertise to companies who are ready for clinical studies. Pharma companies can now evaluate 3M hMTS in their clinical trials as a delivery system for a new drug product or a product line extension," said 3M Drug Delivery Systems vice president Ingrid Blair in a statement. "Keeping patient preference top of mind is key and with this new system, pharmaceutical companies have more options to satisfy patients."
The device is making the rounds at delivery conferences. 3M also touted the device at Boston's Partnership Opportunities in Drug Delivery conference last fall.
A sister product for solid microneedle delivery is being developed. Neither the hollow or solid microneedle device is FDA-approved. A 3M spokesperson said in an email that the company "is actively seeking drug companies to partner with in order to become FDA certified."
In addition, 3M says on its website that it is licensing transdermal drug delivery patches for generic medication, including a 3-day patch for administering the pain relief drug fentanyl and a 24-hour version for controlled release of acetylcholinesterase inhibitor rivastigmine to treat dementia, a condition of Alzheimer's and Parkinson's disease.
Last year, 3M joined forces with Delaware's Invion to develop inhaled drugs for inflammatory airway diseases using its pressurized metered dose inhalation technology.
- read the release
Related Articles
Drug delivery: Fertile ground for pharma-device company partnerships?
3M to develop inhaled airway inflammation treatments with Delaware's Invion
3M sells dry powder inhaler rights to Adamis
Sanofi leads $11M Series A round for needle-free delivery company