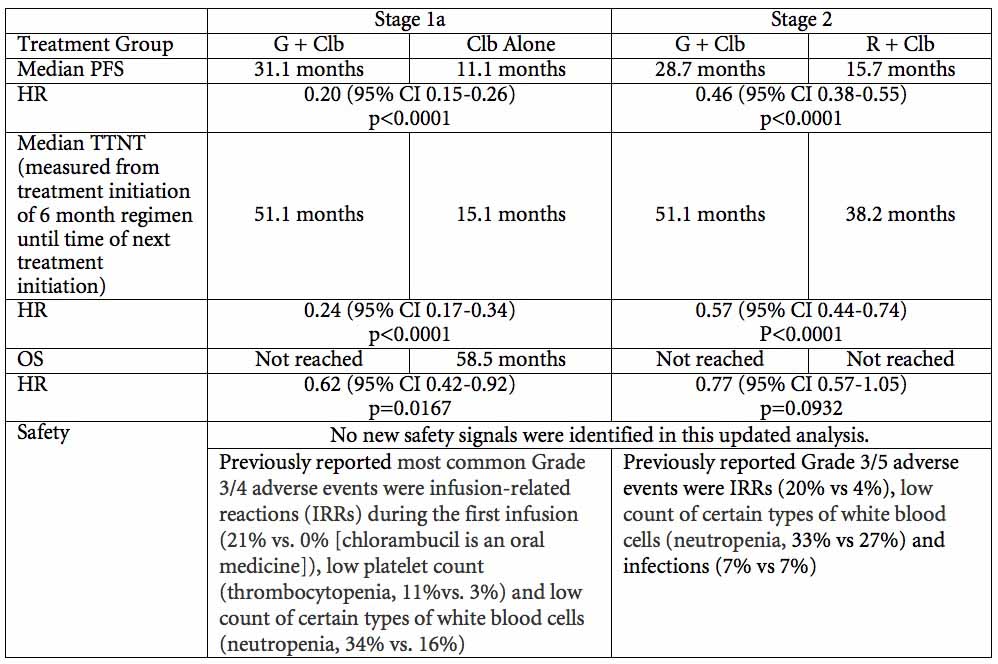
Roche (SIX: RO, ROG; OTCQX: RHHBY) today announced updated data from the pivotal CLL11 study confirming that Gazyva®/Gazyvaro® (obinutuzumab) plus chlorambucil reduced the risk of disease worsening or death by more than half compared to MabThera®/Rituxan® (rituximab) plus chlorambucil (progression-free survival, PFS; HR=0.46, median PFS 28.7 months versus 15.7 months; p<0.0001). New results to be presented at the American Society of Hematology (ASH) Annual Meeting from a secondary endpoint that measured time to next treatment (TTNT) showed that, after completing the set six-month Gazyva/Gazyvaro regimen, people remained treatment-free for nearly four years on average before needing the next treatment for their cancer (TTNT; 51.1 months, including the six month initial treatment period). No unexpected safety signals were observed with Gazyva/Gazyvaro.
"These updated CLL11 data confirmed that Gazyva/Gazyvaro helped people with previously untreated chronic lymphocytic leukaemia live significantly longer without disease worsening or death compared to MabThera/Rituxan," said Sandra Horning, MD, Chief Medical Officer and Head of Global Product Development. "After a fixed course of therapy with Gazyva/Gazyvaro, people remained treatment-free for nearly four years on average. Time free from treatment is an important consideration for a disease like CLL, which occurs in older adults who frequently have other health issues. "
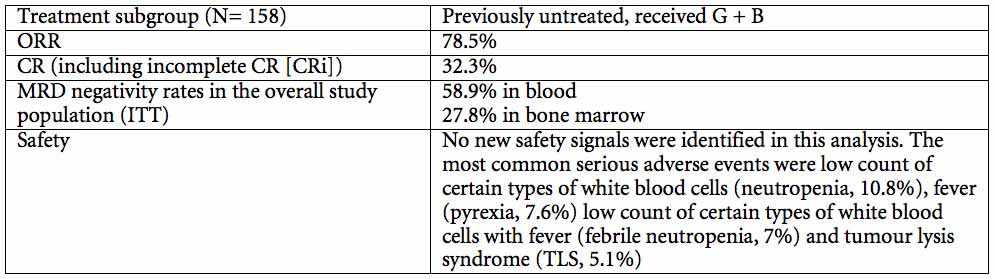
In the GREEN safety study, data from a subgroup analysis showed there were no unexpected safety signals when Gazyva/Gazyvaro was combined with bendamustine. In addition, nearly 80 percent of people responded to treatment with Gazyva/Gazyvaro plus bendamustine (overall response, ORR), and a third of people (32.3 percent) achieved a complete response (CR). A substantial number of people were also minimal residual disease (MRD) negative when measured in the bone marrow or blood (28 percent and 59 percent, respectively), which means no cancer can be detected using a specific test. Overall response rate and MRD were secondary endpoints of the study.
Gazyva/Gazyvaro in combination with chlorambucil is approved in the United States for use in people with previously untreated CLL and in the EU for use in people with previously untreated CLL who have comorbidities making them unsuitable for an intensive therapy (full-dose fludarabine based therapy).
About the CLL11 study
CLL11 is a Phase III, multicentre, open-label, randomised three-arm study investigating the efficacy and safety profile of Gazyva/Gazyvaro plus chlorambucil, MabThera/Rituxan plus chlorambucil and chlorambucil alone in 781 people with previously untreated CLL. Stage 1a (n=589) compared Gazyva/Gazyvaro plus chlorambucil to chlorambucil alone and MabThera/Rituxan plus chlorambucil to chlorambucil alone. Stage 2 (n=663) compared Gazyva/Gazyvaro plus chlorambucil with MabThera/Rituxan plus chlorambucil.
The primary endpoint of the study was PFS and secondary endpoints included overall response rate (ORR), overall survival (OS), complete response rate (CR), response duration, disease free survival (DFS), time to next treatment (TTNT), minimal residual disease (MRD) negativity and safety profile.
The updated analysis from CLL11 will be presented at a poster session on Saturday 5 December at 5:30 pm EST (Abstract #1733).
About the GREEN study
GREEN is an ongoing Phase IIIb safety study. This multicentre, open-label, single-arm study is evaluating the safety and efficacy of Gazyva/Gazyvaro alone or in combination with chemotherapy, including bendamustine, in people with previously untreated or relapsed/refractory CLL. The primary endpoint of the study was safety with secondary endpoints including overall response rate (ORR) and minimal residual disease (MRD) negativity.
The study included a subgroup of people who were previously untreated and who received treatment with Gazyva/Gazyvaro in combination with bendamustine. Data from this subgroup analysis will be presented at an oral presentation on Monday, 7 December at 7:00 am EST (Abstract #493).
About Gazyva/Gazyvaro (obinutuzumab)
Gazyva/Gazyvaro is an engineered monoclonal antibody designed to attach to CD20, a protein found only on B-cells. Gazyva/Gazyvaro is designed to attack and destroy targeted B-cells both directly and together with the body's immune system.
Gazyva/Gazyvaro is currently approved in more than 60 countries in combination with chlorambucil, for people with previously untreated CLL. The approval was based on the CLL11 study, showing significant improvements with Gazyva/Gazyvaro plus chlorambucil across multiple clinical endpoints, including progression-free survival (PFS), overall response rate (ORR), complete response rate (CR), and minimal residual disease (MRD) when compared head-to-head with MabThera/Rituxan plus chlorambucil.
Gazyva is marketed as Gazyvaro in the EU and Switzerland.
Gazyva/Gazyvaro is being studied in a large clinical programme, including the Phase III GOYA and GALLIUM studies. GOYA is comparing Gazyva/Gazyvaro head-to-head with MabThera/Rituxan plus CHOP chemotherapy in first line diffuse large B-cell lymphoma (DLBCL) and GALLIUM is comparing Gazyva/Gazyvaro plus chemotherapy followed by Gazyva/Gazyvaro maintenance head-to-head with MabThera/Rituxan plus chemotherapy followed by MabThera/Rituxan maintenance in first line indolent non-Hodgkin's Lymphoma (NHL). Additional combination studies investigating Gazyva/Gazyvaro with other approved or investigational medicines, including cancer immunotherapies and small molecule inhibitors, are planned or underway across a range of blood cancers.
About CLL
CLL is the most common type of leukaemia in the Western world. It accounts for 25-30% of all leukaemias1, equating to over 80,000 cases of CLL being diagnosed each year2.
About Roche in haematology
For more than 20 years, Roche has been developing medicines that redefine treatment in haematology. Today, we're investing more than ever in our effort to bring innovative treatment options to people with diseases of the blood. In addition to approved medicines MabThera®/Rituxan® (rituximab) and Gazyva®/Gazyvaro® (obinutuzumab), Roche's pipeline of investigational haematology medicines includes an anti-PDL1 antibody (atezolizumab/MPDL3280A), an anti-CD79b antibody drug conjugate (polatuzumab vedotin/RG7596), a small molecule antagonist of MDM2 (idasanutlin/RG7388) and in collaboration with AbbVie, a small molecule BCL-2 inhibitor (venetoclax/RG7601/GDC-0199/ABT-199). Roche's dedication to developing novel molecules in haematology expands beyond oncology, with the development of the investigational haemophilia A treatment emicizumab (ACE910).
About Roche
Headquartered in Basel, Switzerland, Roche is a leader in research-focused healthcare with combined strengths in pharmaceuticals and diagnostics. Roche is the world's largest biotech company, with truly differentiated medicines in oncology, immunology, infectious diseases, ophthalmology and neuroscience. Roche is also the world leader in in vitro diagnostics and tissue-based cancer diagnostics, and a frontrunner in diabetes management. Roche's personalised healthcare strategy aims at providing medicines and diagnostics that enable tangible improvements in the health, quality of life and survival of patients. Founded in 1896, Roche has been making important contributions to global health for more than a century. Twenty-nine medicines developed by Roche are included in the World Health Organization Model Lists of Essential Medicines, among them life-saving antibiotics, antimalarials and chemotherapy.
In 2014, the Roche Group employed 88,500 people worldwide, invested 8.9 billion Swiss francs in R&D and posted sales of 47.5 billion Swiss francs. Genentech, in the United States, is a wholly owned member of the Roche Group. Roche is the majority shareholder in Chugai Pharmaceutical, Japan. For more information, please visit roche.com.
All trademarks used or mentioned in this release are protected by law.
References
1 Thomas, D (2010) How common is CLL? Chronic Lymphocytic Leukemia Frequently Asked Questions. http://cllfaq.info/general.html (URL accessed 30.08.13).
2 Globocan (2012) Estimated incidence, mortality and 5-year prevalence:both sexes: http://globocan.iarc.fr/old/summary_table_pop-html.asp?selection=224900&title=World&sex=0&type=0&window=1&sort=0&submit=%C2%A0Execute (URL accessed 27.10.15)