 After recalling 18 million vials last January of a painkiller commonly used in surgery, sterile injectables maker Hospira indicated that it was on top of the problem. But the company now says new manufacturing issues have cropped up that have led it to recall more than 20.7 million more vials of ketorolac, a drug on the FDA shortage list.
After recalling 18 million vials last January of a painkiller commonly used in surgery, sterile injectables maker Hospira indicated that it was on top of the problem. But the company now says new manufacturing issues have cropped up that have led it to recall more than 20.7 million more vials of ketorolac, a drug on the FDA shortage list.
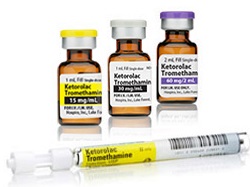
According to the most recent FDA Enforcement Report, Hospira ($HSP) is recalling 15,477,475 vials of ketorolac tromethamine in a 30-mg dosage of which about 12.5 million vials have the Hospira label and more than 3 million vials sport a label for customer Novaplus. It also is recalling 5,465,500 vials in a 60-mg dosage, with more than 5.2 million with the Hospira label and 250,600 with the Novaplus label. The Lake Forest, IL-based company tagged the recall to crystallization of calcium salt. The drug, a nonsteroidal anti-inflammatory, has been on the FDA shortages list since at least early this year. Fresenius Kabi has indicated it has product available in different doses but suggests providers check with wholesalers about what is on hand.
On top of that, the drugmaker added to the national shortage of saline solution by recalling 314,600 bags of 0.9% sodium chloride because of possible leaks in bags.
Hospira, in an emailed statement, said the company "remains committed to the highest standards of product and manufacturing quality, and takes very seriously our responsibility to ensure we follow regulations and company operating procedures."
It went on to say, "In the last few years, Hospira has invested more than $1 billion across the company to help ensure high-quality products are available to patients in sustainable supply. We are proud of our progress, but our work is not done. We remain dedicated and focused across our global network to ensure that we are meeting all the requirements of global regulatory bodies, and executing on the commitments that we have made to ensure sustainable compliance across our entire manufacturing footprint."
Pfizer ($PFE) will be looking for those investments to pay off, since it announced in January that it would buy Hospira for $15 billion and the assumption of $2 billion in debt. Pfizer, of course, is aware of the quality issues that Hospira has struggled with for years. Its warning letters for plants in the U.S. and India, and more recently in Australia and Italy, have been highly publicized, as have its frequent recalls. Hospira has had more class I recalls than any other drugmaker in the past several years, 34 going back to 2012. But Pfizer, which expects to close the deal this year, has said it has evaluated Hospira's operations and is happy with its decision to add it to its operations.
- the recall notices are here, here and here
Special Report: Top Class I drug recallers