 |
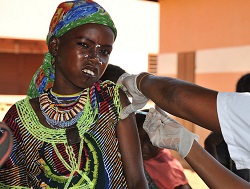
| A girl receives the MenAfriVac vaccine in Benin--Courtesy of the WHO/Rodrigue Barry |
The affordable meningitis A vaccine, MenAfriVac, has been given to millions of people in Africa's so-called "meningitis belt," and is about to be administered to even more. The World Health Organization approved the shot, previously approved for people aged one to 29, for use in the routine immunization of infants less than one year old in sub-Saharan Africa.
The approval was announced Friday by the Meningitis Vaccine Project (MVP) and Serum Institute of India (SIIL), which manufactures the vaccine. The MVP is a partnership between the WHO and the global nonprofit, PATH.
MVP partnered with Serum in 2004 to develop an affordable vaccine that was tailor-made for use against meningitis A in sub-Saharan Africa. According to a WHO release, the vaccine was developed in record time and at one-tenth the cost of a typical new vaccine.
It was introduced in Africa in 2010, and since then, more than 215 million people have been vaccinated in 15 "meningitis-belt" countries. The affected area stretches from Senegal in the west to Ethiopia in the east, and includes Benin, Burkina Faso, Cameroon, Chad, Côte d'Ivoire, Ghana, Mali, Niger, Mauritania, Nigeria, Sudan, Togo and Gambia.
MenAfriVac's efficacy was confirmed "in a major way" in Chad in 2012. Researchers found that transmission and incidence of meningitis A had dropped 90% following vaccination. But while results have been dramatic, there is still work to be done.
"Initial mass vaccination campaigns with MenAfriVac® have been highly effective in reducing the number of meningitis A cases," said Dr. Marie-Pierre Préziosi, director of MVP, in the release. "But epidemics will return when rising numbers of unprotected newborns become a larger proportion of the total population over time."
WHO's decision means that most of the population will continue to be protected as infants are routinely immunized against meningitis A. The organization is already working with African countries on a transition from mass campaigns to routine immunization, it said in the release. Seven countries are on track to introduce MedAfriVac in their routine system as early as 2015. Mass campaigns will continue in the other countries.
Before MenAfriVac, meningitis vaccines were quadrivalent and expensive, Reuters reports. African leaders wanted something "better," and cheaper too.
"Developing the MenAfriVac vaccine fit exactly Serum's ingrained philosophy of bringing down prices of vaccines so that under-privileged children of the world are protected," said Dr. Cyrus Poonawalla, CEO of the Pune, India-based SIIL.
In fact, in a June 2011 trial, MenAfriVac beat out pricier vaccines from other companies, including GlaxoSmithKline ($GSK). After four weeks of treatment, 96% of the 600 African infants and toddlers who were given MenAfriVac had antibodies in their blood, compared with 64% of those who had received Glaxo's vaccine.
MenAfriVac, Reuters reports, is the first vaccine made specifically for Africa, costs 50 cents a shot, and doesn't need constant refrigeration.
- read the release
- get more from Reuters