 |
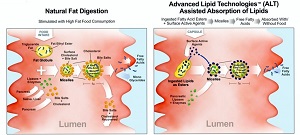
| Comparison of natural fat digestion and ALT-assisted absorption of lipids--Courtesy of Sancilio (Click to enlarge) |
Sancilio Pharmaceuticals announced its plans for an IPO on the Nasdaq worth up to $86 million in an SEC filing.
The company is advancing four lead candidates based on its Advanced Lipid Technologies drug delivery platform, which it says enables absorption of drugs in the absence of healthy intestines, bile or normal gastric enzymes. It also avoids the "food effect," meaning absorption occurs regardless of whether food is present in the body.
ALT increases the solubility of insoluble or poorly absorbed compounds like hormones, omega-3 ethyl esters and other lipid-based or lipid-soluble compounds, Sancilio says on its website. Details about the mechanics of the platform are sparse, but the formation of micelles plays a crucial role. They consist of an aggregation of lipid molecules that arrange themselves in spherical formation so that the hydrophilic heads are facing the surface and shielding the molecule's hydrophobic tails from the external environment. Other molecules can be encapsulated within the structure.
Sancilio's severe hypertriglyceridemia candidate is expected to begin two pivotal pharmacokinetic trials soon, according to the SEC filing. And the company intends to file an Investigational New Drug Application (IND) for its sickle cell anemia and short bowel syndrome candidates by the end of the year or first quarter of 2016. Both have been granted an orphan drug designation. Also expected by the end of the year is an IND for its nonalcoholic fatty liver disease candidate.
All of the drugs' lipophilic active pharmaceutical ingredients are already proven or approved, which should result in an easier regulatory process. Three of the candidates are administered via soft-gelatin capsules, while that for short bowel syndrome (SC403) in premature infants is added to breast milk or baby formula.
The company explained its drug delivery in the SEC filing, writing "It appears that when SC403 is mixed with breast milk or baby formula, stable micelles are immediately formed which encapsulate the fatty acids of SC403, as expected, as well as the other nutrients that are present in the breast milk or baby formula. These micelles are then ingested by the baby and the fatty acids that comprise SC403 and the other nutrients now encapsulated in the micelle are able to pass through into the intestinal lining."
Sancilio believes the candidate will increase absorption of fatty acids and other vital nutrients, and think it may be able to contribute to the growth of the intestinal cells.
Sancilio is also an active partner with other pharma players including Mylan ($MYL) and TherapeuticsMD. "We are currently developing seven Abbreviated New Drug Application, or ANDA, drug products for third parties, four of which have been submitted and are awaiting FDA approval. We are also developing an additional four 505(b)(2) NDA drug products for third parties utilizing our proprietary technologies," the SEC filing says.
The company, which had revenues of about $24.5 million in 2013 and 2014, also makes over-the-counter and behind-the-counter dietary supplements under the Ocean Blue brand, as well as prenatal vitamins and dental health products.
Sancilio racked up a combined loss of about $7.7 million in 2013 and 2014.
Besides the ALT delivery platform, the company believes its strength lies in manufacturing drug delivery technology. "We believe there are a limited number of other companies in the United States that can produce soft gelatin capsules, and, to our knowledge, none of these use our proprietary methods," the SEC filing says, adding, "We believe that our ability to use these proprietary methods in the manufacture of our proprietary product candidates will provide us with a competitive advantage and be a barrier to entry for other pharmaceutical companies."
- here's more from Renaissance Capital
- read the SEC filing