 |
| "Robotic pill"--Courtesy of Rani Therapeutics |
Oral biologics are a drug delivery puzzle, so far unsolved, but have tremendous commercial potential. Typically, experimental oral formulations of the protein-based drugs degrade in the enzyme of the small intestine, limiting absorption, which is why none have made their way to the market. Biologic drugs for diseases like diabetes and rheumatoid arthritis remain injectable for now, but Rani Therapeutics' novel oral biologic delivery system just earned a partnership with pharma bigwig Novartis ($NVS), suggesting that the San Jose startup is on the way to cracking the code.
The companies will conduct feasibility studies on the administration of Novartis' proprietary biologics into the bloodstream using Rani's so-called "robotic pill." The feasibility study will last for 18 to 24 months, after which Novartis has the right to enter in a more extensive collaboration with Rani and license the pill for specific uses, according to a release.
"We understand the magnitude of the problem we are pursuing, and we are confident that our approach has the potential to radically change the way biologics are administered to patients," said Mir Imran, CEO of Rani Therapeutics, in a statement. "We are delighted to be embarking on this journey with Novartis, one of the world's largest and most successful pharma companies."
Novartis also made an equity investment in Rani via its Series C financing round worth a total of more than $25 million. Previous investors in the company include Google Ventures, InCube Ventures and VentureHealth. Imran is an accomplished entrepreneur, having formed more than 20 companies, which have been bought by the likes of med tech titan Medtronic ($MDT), according to the The Wall Street Journal. He also owns more than 300 patents.
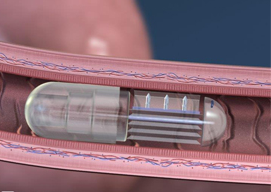
The pill's drug delivery paradigm has a strong mechanical (rather than chemical) component, earning it the nickname robotic pill.
Following the robotic pill's passage through the stomach, the slightly acidic fluids of the small intestine dissolves the pill's casing, as well as a nanoscale valve. Once the valve is released, previously separated citric acid and sodium bicarbonate mix to form carbon dioxide, which in turn inflates a small balloon tipped with needlelike structures made of sugar.
The needles rise on the edge of the balloon and embed themselves in the small intestine. No pain is felt because the organ doesn't have any pain receptors. Next, the needles detach from the robotic pill and release drugs into the proximate blood vessels while dissolving in the body. Meanwhile, the remainder of the pill passes through the body.
The robotic pill still has to overcome regulatory hurdles, which could add time to the FDA review of any pharma candidate that it delivers. However, the FDA has allowed two other pills into the market that perform mechanical functions, sort of like small medical devices.
Given Imaging and its PillCam for noninvasive imaging of the colon was acquired by Covidien, while Proteus Digital Health's electronic pill for tracking medication usage has received hundreds of millions in VC financing. Unlike those products, the robotic pill does not contain any electronic components.
Johnson & Johnson ($JNJ) recently struck a partnership with another oral biologics aspirant, Applied Molecular Transport, which claims to have created a drug delivery method that shuttles proteins across the intestinal epithelia based on the behavior of naturally occurring microbes.
Both partnerships are in the preclinical stage, indicating that oral biologics are possible, but not any time soon. The first company to cross the finish line is likely in for a big payday.
- read the release