 |
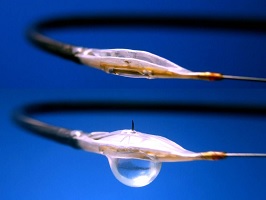
| Mercator's Bullfrog and Cricket microinfusion catheters.--Courtesy of Mercator |
California's Mercator MedSystems has begun treating its first patient in an ischemia trial of its microinfusion device that delivers an anti-inflammatory steroid.
Mercator's Bullfrog is designed to reopen arteries below the knee for patients with critical limb ischemia, which causes inadequate blood supply to that area of the body. Along with an angioplasty, the Bullfrog delivers a dose of the steroid dexamethasone, which decreases the buildup of scar tissue in the vessel, helping to reduce cases of restenosis.
Mercator is testing the CE-marked and FDA-cleared device at the University Heart Center in Bad Krozingen, Germany, according to a release, and is expecting to enroll up to 120 patients. It follows two trials in above-the-knee arteries.
"This is a very important milestone for the company," Mercator CEO Trent Reutiman said in a statement. "There is an unmet need in delivering a reliable drug therapy to keep arteries open below the knee. Our results in above-knee arteries from our DANCE-Pilot study and our most recent experience from our larger, 285 patient DANCE trial provide us with confidence that our micro-infusion technology can place (the) drug directly where it is needed and will have a strong benefit."
Bullfrog is one of two Mercator microinfusion catheters, a lineup that also includes its Cricket device. Back in 2013, the company had picked up $18.5 million in equity financing from two rounds, funding it used to move forward with these trials.
- here's the release