Everolimus has eclipsed paclitaxcel as the drug of choice in drug-eluting stents, which are a definitive advance over bare metal stents. The third generation of stents is on the way. Bioresorbable stents are absorbed by the body within a year. But the advantages of localized drug delivery are not lost, as the market leader elutes everolimus.
Is that the optimal drug for the new class of stents? Perhaps the right choice is under development by nonprofit contract research organization CBSET of Lexington, MA. They're testing a sirolimus analog called corolimus.
The main benefit of corolimus is fast absorption, which enables stent degradation in weeks, not months, reducing the risk of stent thrombosis, or the formation of blood clots around the device. Another problem with long-lasting or permanent stents is late-lumen loss, or the reformation of plaque at the site of the implantation.
"We developed and evaluated a cross-linked omega-3 fatty acid (O3FA)-based coating that is 85 percent absorbed and elutes 97 percent of its Sirolimus analog (Corolimus) load within eight days of implantation. This fast absorption allows for implanted stent struts to be quickly endothelialized, thereby reducing the risk of late thrombosis with minimal and stabilizing late-lumen loss," said Dr. Keith Faucher, the director of research and analytical chemistry at Maquet Vascular Systems, in a release.
Abbott's ($ABT) Absorb BVS is the market leader in the bioresorbable stent arena, with sales around $132 million internationally in 2014, according to Global Data. U.S. sales are projected to hit $96.3 million by 2017 in the U.S. But that estimate assumes an FDA approval in 2016.
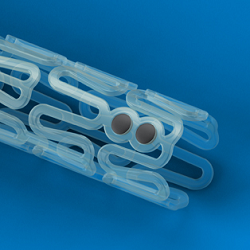 |
| Abbott's Absorb bioresorbable stent--Courtesy of Abbott |
While the device has shown statistical non-inferiority to Abbott's drug-eluting Xience stent, an editorial in the New England Journal of Medicine said that "most clinicians in everyday practice would not accept this degree of difference between two stents in their catheterization laboratories. This means that the clinical relevance of the finding of statistical non-inferiority is open to question."
Indeed, a close inspection of the BVS data that went beyond the top line inflamed passions on both sides at the TCT conference, and should make for an interesting advisory panel discussion at the FDA.
But Abbott should find recent comments made by Dr. Jeffrey Shuren (the head of FDA's device arm) at the AdvaMed conference reassuring. He said that the first iteration of a paradigm shifting device (such as a bioresorbable stent) is often a bit underwhelming, but its approval is often needed to ensure patients can benefit from subsequent improvements down the line.
The CBSET research suggests the improvements could come in the form of improved drug formulations and delivery, rather than purely mechanical enhancements. Additional details can be found in a paper in the Annals of Biomedical Engineering.
"This study represents a significant medical milestone in the ongoing development of therapies to treat coronary artery disease," said study author and CBSET co-founder Dr. Elazer Edelman in a statement. "Now, the composition of Sirolimus-eluting stent (SES) coatings can be designed for optimal biocompatibility and bioabsorption rather than drug-elution alone. This paradigm shift creates an opportunity for the pharmaceutical and medical technology industries to use a range of biocompatible materials that may not have otherwise been considered."
According to the study abstract, "the long held assumption that sustained drug elution from stent coatings over weeks to months is imperative for clinical efficacy has limited the choice for stent coating materials."
It seems likely that a Big Pharma's expertise and clinical trial know-how will be needed in order to overturn that assumption and take a new limus-based compound past the FDA. Everolimus is exclusively licensed to Abbott and Boston Scientific ($BSX) by Novartis ($NVS). Other players use slightly different formulations of limus.
Meanwhile, Medtronic ($MDT) is testing a novel drug delivery methods in investigational, nonbioresorbable drug-filled stent. Its administration of sirolimus is designed to reduce the body's exposure to polymers.
- read the release
- here's the paper abstract
Special Report: Leaders emerge in the race for the first U.S. bioresorbable stent