 |
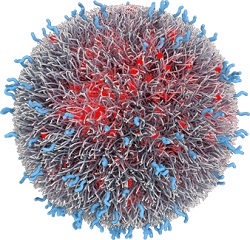
| Accurin nanoparticle for cancer drug delivery--Courtesy of Bind |
Cambridge, MA's Bind Therapeutics announced that a Phase II squamous histology non-small cell lung cancer cohort treated with its one of its candidates will progress to the next stage and complete enrollment at 40 patients, while the KRAS mutant arm of the trial will be terminated.
The patients were treated with non-small cell lung cancer candidate BIND-014, which utilizes the company's signature Accurins nanoparticle drug delivery platform.
In October, the two arms reached the 20-patient enrollment mark, "triggering a planned evaluation of the data against pre-specified gating criteria for continuation to stage 2." The criteria included 6-week disease control rate, tolerability, and, in the case of the successful arm, the inspection of updated overall survival data from a previous clinical trial.
In the squamous histology cohort, the 6-week disease control rate was 25%. The final median overall survival among 9 squamous histology patients from a previous trial of the candidate was 11.1 months, and their one-year survival rate was 44%. Meanwhile, the unsuccessful KRAS mutant cohort had a 6-week disease control rate of 17.4%.
"Based on preliminary data from (the) iNSITE 1 (trial) and confirmed median overall survival data from the 005 trial, we believe that BIND-014 may be ideally suited to broaden the impact of immuno-oncology approaches in the treatment of solid tumors," said Bind CEO Andrew Hirsch in a statement. "Our next steps are to complete enrollment in the squamous cohort of iNSITE 1 in early 2016 and, in parallel, begin designing a trial in combination with a checkpoint inhibitor that we intend to pursue contingent upon final iNSITE 1 results, potentially through new collaborations with development partners."
Bind's nanoparticles are delivered via infusion and consist of the drug payload, a controlled-release polymer matrix, a polyethylene glycol "hydration shell" to protect the particle from the immune system, and targeting ligands for localized delivery, which can be small molecules, peptides, antibodies or antibody fragments, according to the company's website. The company says the platform achieves prolonged circulation in the bloodstream, targeted delivery and controlled and timely release.
Factors like polymer length and surface charge can be tweaked to meet the specifications of the payload and therapeutic area, according to Hirsch. "It's a classic Langer approach, which is, let's take an engineering approach to a biological problem," Hirsch said in a prior interview, referring to drug delivery guru Robert Langer of MIT, who is a Bind co-founder and serves on the company's board of directors.
- read the release