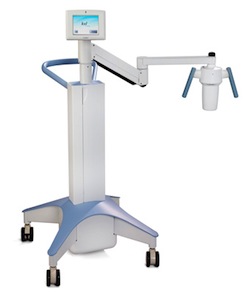
 |
| Avedro's KXL system--Courtesy of Avedro |
Waltham, MA's Avedro announced that it has raised $32 million in financing to expand global distribution of its KXL corneal cross-linking platform for the delivery of drops of riboflavin into the eye of patients suffering from vision impairment due to keratoconus.
The investors are hoping for a better outcome at the FDA than in March 2014, when the regulator rejected the combination product, which is already sold in more than 60 countries. As well as the KXL device, the platform consists of the specially formulated riboflavin (or vitamin B-12) eye drops. The company expects to hear word on an FDA approval (or rejection) by April 2016. A yes would make Avedro the first to offer an FDA-approved therapeutic for progressive keratoconus and corneal ectasia following refractive surgery.
The KXL system cuts treatment time from 1 hour to 14 minutes or less, the company boasts. It does so by accelerating what's known as cross-linking by exposing the cornea to ultraviolet light for 4 minutes following the application of the riboflavin. According to the company website, doing so makes the riboflavin fluoresce, "leading to the formation of bonds between collagen molecules or collagen cross-linking."
During the standard cross-linking procedure, the ultraviolet light exposure takes 30 minutes, and the riboflavin presoak takes 30 minutes, while KXL says on its website that it presoaks the active ingredient in 10 minutes. The company says it is a first-line treatment aimed at stopping or reducing progression of the disease, which causes progressive vision loss and can lead to the need for corneal transplantation.
In addition, Avedro said the financing will help it commercialize the Mosaic System outside of the U.S. The device performs the so-called PiXL accelerated cross-linking procedure to treat myopia. PiXL does not involve the use of Riboflavin drops.
The round was led by VCs InterWest and OrbiMed, as well as several other existing private financial investors.
"The Avedro corneal cross-linking technology is eagerly awaited by many in the eye care community," InterWest general partner Dr. Gil Kliman said in a statement. "This new investment will provide the resources needed to commercialize in the United States, if FDA approval is received, as well as help fund the longer term vision of PiXL as a non-invasive refractive procedure."
- read the release
- here's FierceMedicalDevices' take
Special Report: The Biggest Med Tech VC Investments of Q1