 |
| Reversir molecule (red)--Courtesy of Alnylam |
Alnylam ($ALNY) unveiled two new additions to its RNAi drug delivery arsenal at the meeting of the Oligonucleotide Therapeutics Society in Leiden, Netherlands, as it strives to be first biotech to commercialize a therapeutic that leverages the naturally occurring pathway.
At the meeting, senior scientist Ivan Zlatev described the company's internally developed Reversir molecules for the reversal of siRNA silencing activity. Or as COO Barry Greene put it during an interview, "What our scientists invented was a technology to reverse the pharmacology of an RNAi-mediated therapeutic program."
Greene said the breakthrough is a feature that makes Alnylam's RNAi drug delivery platform more powerful, although he stressed the company has not yet created a Reversir molecule for any of its specific programs or candidates.
Subcutaneous injection of a Reversir molecule is designed to stop an Alynlam RNAi therapeutic from achieving its normal biologic effect on the body, Greene said, citing data presented at the conference showing that the molecules were well-tolerated and reversed siRNA-conjugate activity in rodents after about 10 days.
Hypothetical benefits of Reversir molecules could include the ability to stop drug activity before a surgery or after an inadvertent injection, Greene said, adding "I can imagine us integrating this into our programs over the next couple of years."
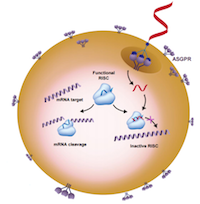
Greene explained the Reversir molecules bind to antisense strands in functional RNAi-induced silencing complexes (RISCs) to prevent mRNA cleavage, enabling the normal "DNA makes RNA makes protein" process to continue.
In addition, Alnylam presented data on its bis-RNAi conjugate molecules for the simultaneous silencing of two genes with a single molecular entity. The drug delivery technology delivers two strands of siRNA to the cell, potentially improving the therapeutic benefits of Alnylam candidates.
Alnylam evaluated two designs of bis-RNAi with different linker structures, and has developed a screening system to evaluate various conjugates, according to a poster on its website. Early results of bis-RNAi on human whole blood assays and mice models show promising signs of efficacy and demonstrate that molecules do not induce a cytokine response, the poster says.
The company expects to advance its first bis-RNAi candidate as soon as next year, and says the platform advance has applications to its cardio-metabolic and hepatic infectious disease therapeutic areas.
Although neither breakthrough has worked its way into a particular Alnylam candidate yet, the company is advancing therapies through its pipeline that use its second-generation "ESC" GalNAc conjugate technology, including a Phase III hemophilia candidate. And during the conference, Alnylam presented preclinical data on one of its ESC candidates for TTR-mediated amyloidosis.
Greene said that the second generation platform improves patient safety, efficacy and convenience because it enables stronger drug potency and less frequent subcutaneous dosing.
Alynlam's platform enhancements come as RNAi is threatened with becoming a victim of its own success, as clinicians and investors impatiently await a clinical and economic payoff in the form of a successful commercial therapeutic.
The naturally occurring paradigm's discovery in 1998 earned scientists Andrew Fire and Craig Mello a Nobel Prize in 2006 to great fanfare, and subsequent investments from Big Pharmas Roche ($RHHBY), Novartis ($NVS) and Merck ($MRK), all of whom subsequently canceled their programs due to the difficulty of drug delivery of RNAi candidates across cell membranes.
Greene defended his company last year by going on the offensive, telling FierceDrugDelivery that Big Pharma "has been a miserable barometer of high-impact technologies" and "has never been able to innovate" after Novartis' RNAi pullout sent Alnylam's stock down 20% amid a renewed wave of nervousness.
As another sign of decreased (popular) interest, the Economist magazine notes that Google searches for "RNAi" have been steadily decreasing since peaking around 2005. But pointing to the strong pipelines and innovative drug delivery strategies of Alynlam and competitors Dicerna and Benitec Biopharma, the article concludes that the herd likely gave up on RNAi a little too early.
- read the release about the two new features | here's more about Alnylam's preclinical candidate (ALN-TTRsc02)
- here are presentation slides about Reversir molecules | and a poster about bis-RNAi
- here's The Economist's take on RNAi
Special Report: FierceDrugDelivery's 10 biggest partnerships in drug delivery - Sanofi-Alnylam