Can MannKind ($MNKD) really resurrect its inhaled insulin Afrezza? Sanofi ($SNY), a diabetes powerhouse, tried and failed to make a market for the product. Its drawbacks remain. But as the Wall Street Journal points out, MannKind CEO Matthew Pfeffer thinks Sanofi bungled, and it's all down to pricing and reimbursement to turn things around.
 Sanofi ended its marketing deal on Afrezza last month, bailing out of a $925 million arrangement just $200 million or so to the worse, execs told the WSJ. The French drugmaker tried to turn some early patient enthusiasm into a broad base of converts, but in the end, decided that the obstacles were too great. The drug only managed to bring in $7.5 million last year.
Sanofi ended its marketing deal on Afrezza last month, bailing out of a $925 million arrangement just $200 million or so to the worse, execs told the WSJ. The French drugmaker tried to turn some early patient enthusiasm into a broad base of converts, but in the end, decided that the obstacles were too great. The drug only managed to bring in $7.5 million last year.
Those obstacles are indeed daunting: To get access to the drug, patients have to undergo lung testing with a spirometer, which isn't a common piece of physician-office equipment. The testing was "a major hurdle," JPMorgan analyst Cory Kasimov said last year, and that requirement, along with the FDA's cautionary language about breathing difficulties, made already wary doctors even more so.
The truth is, Sanofi may have overestimated the appeal of an inhaled product over an injected one. It may have over-attributed the failure of Pfizer's ($PFE) inhaled insulin, Exubera, to the unwieldy design of its Pringles-size delivery device. But even among the 6,000 patients who wanted the inhaled product, only 35% stayed on the drug, a Sanofi spokeswoman told the WSJ.
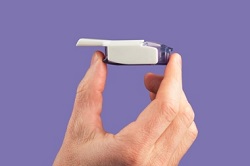
MannKind appears set to ignore those statistics. Pfeffer maintains that patients want a needle-free option and sees Afrezza's whistle-shaped device as the answer. To him, Afrezza would have been fine if it had been priced lower and earned better coverage with payers.
After Sanofi ended the partnership, Pfeffer said he'd look for a new partner and cut the price of the drug. Priced at a premium to injectables, Afrezza sits on most payers' third tier for reimbursement, the WSJ says, and Pfeffer says pricing and reimbursement "dramatically outweigh other factors" that hampered the new launch.
Future Afrezza plans have to involve educating patients and doctors, as well as improving patient access, Pfeffer said on a call with analysts last month. He believes patients really are clamoring for a drug like Afrezza, even with all the evidence to the contrary. Why? "The real world experience of Afrezza users ... is everything we hoped it would be," Pfeffer said during the call, vowing, "this is not the end of the line for Afrezza or MannKind by any means."
Pfeffer has until July 4 to bring in a new partner before Sanofi is completely out of the picture. The Afrezza commercialization pact officially terminates no later than Independence Day.
- see the WSJ piece (sub. req.)
Special Report: Top 10 best-selling diabetes drugs of 2013