 |
| Courtesy of Parhamr, CC public domain |
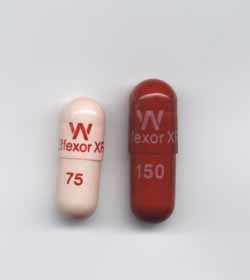
The largest drugstore chains have been in a legal battle with Pfizer ($PFE), accusing it of conspiring to keep generics of its antidepressant Effexor XR out of their stores for years. Now they will have to deal with losing some of the real thing. Pfizer is recalling three lots of the drug after a pharmacist discovered a capsule of one of Pfizer's heart pills in an Effexor XR bottle, a potentially fatal combo.
The drugmaker is recalling two lots of different doses of Effexor XR and one lot of its Greenstone-branded Venlafaxine, its branded generic version. The drugmaker said the risk that any other bottles might be affected is slim, but it decided to recall the three lots of Effexor XR as a precaution. All three were run on the same packaging line. Pfizer told Fox News that the three lots total about 104,450 bottles, and about 65,800 have already hit pharmacies.
The heart drug found in an Effexor XR bottle was Tikosyn, a med used to treat irregular heartbeats. There is the potential for a patient prescribed Effexor XR who took a Tikosyn by mistake to have a fatal reaction, the drugmaker cautioned.
The largest pharmacy chains including CVS Caremark ($CVS), Rite Aid ($RAD) and Walgreen ($WAG) a couple of years ago sued Pfizer's Wyeth unit and Teva Pharmaceutical Industries ($TEVA) saying they colluded to delay generic versions of the blockbuster antidepressant. That delay caused them to pay too high a price for the drug, they claim. The FTC asked to join the lawsuit last year.
Effexor XR's original patent expired in 2008, but Effexor XR generics didn't make their debut for two years afterward, under a 2006 patent settlement with Teva. The drugstores claim they overpaid for Effexor XR because of the drugmakers' scheming. Effexor XR brought in $2.5 billion a year as long as the generics were delayed, the drugstores say, but dropped 61% in the first year that generics were available. Teva and Pfizer denied they did anything wrong.
- here's the FDA announcement
- read more from Fox News