 |
| FiercePharma file photo |
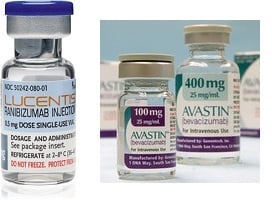
Swiss drugmakers Roche ($RHBBY) and Novartis ($NVS) have stirred up a hornet's nest in Europe over their efforts to steer doctors away from off-label use of cancer drug Avastin in favor of the pricier Lucentis for treating sight issues. They have been fined by the anticompetition authority in Italy, where prosecutors are also taking a look, and now French regulators say they are in the midst of a probe of their own.
Chances are more governments will pile on, Bryan Garnier & Co. analyst Eric Le Berrigaud told Bloomberg. "The subject isn't closed," the Paris-based analyst said, and "in all likelihood" will be followed by investigations by other countries.
Henri Pitron with the Finance Ministry told Bloomberg that the agency started looking into the pricing matter at the end of 2012 at the request of French Health Minister Marisol Touraine. He said the investigation is "ongoing."
The Italian Competition Authority last week fined Roche and Novartis combined penalties of €182.5 million ($253 million), claiming they had catalyzed sales of the two drugs by blocking distribution of Avastin in favor of the more expensive Lucentis. Armed with evidence gathered in the antitrust probe, Italian prosecutors launched an investigation of their own. They claim they discovered "numerous messages" between the two companies about how to persuade doctors and hospitals to use the pricey Lucentis. The companies denied they did anything wrong and said they would appeal the fines.
Avastin is not approved for wet age-related macular degeneration, while Lucentis is. But because the two drugs work similarly to restrict blood vessel growth, some researchers have found the treatments more or less equally effective. That has led some doctors to use Avastin off label for the condition. The savings are significant: A Lucentis injection in Italy costs €900 (about $1,230) compared with €81 ($111) for Avastin. Italian regulators estimate that blocking access to Avastin for use in the eye cost the country's health system €45 million ($62 million) in 2012 and could end up costing €600 million ($824 million) or more per year in the future.
The actions come as government-financed health systems in Europe are looking for ways they can trim their growing costs. Bloomberg points out that the French health minister has talked about allowing the temporary use of unapproved drugs for "economic reasons," while in Italy officials are looking at legislation that would allow off-label uses to save money.
- here's the Bloomberg story
Related Articles:
Italian prosecutors jump into Roche, Novartis case with fraud probe
Novartis' Lucentis gets its fourth approval in E.U.
Italy levies eye-opening antitrust fines against Roche, Novartis over Lucentis
Italian antitrust agency probes alleged Roche-Novartis cartel
Novartis balks at Eylea-vs.-Lucentis data