 |
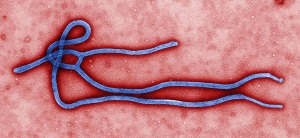
| Ebolavirus under an electron microscope--Courtesy of CDC |
SINGAPORE--The head of Guinea's Ebola-response program wasted no time announcing approval of Fujifilm's Avigan (favipiravir) influenza drug to treat early cases of Ebola in the West African nation.
Program Coordinator Sakoba Keita said the drug, developed by Fujifilm's Toyama Chemical unit, would be available only in three special Ebola-treatment centers in the country and possibly two additional centers later.
Using Avigan to treat patients with low to moderate levels of the virus in their blood will be limited to those clinics. The drug would not be made available to patients in hospitals, either public or private, the government said. "It takes training of health workers on the use of this drug," the announcement said.
 The government acted soon after Toyama disclosed preliminary results from Phase I clinical trials showing the drug trimmed the mortality rate in early stages to 15% from 30%. The patients also reported no adverse side effects from Avigan.
The government acted soon after Toyama disclosed preliminary results from Phase I clinical trials showing the drug trimmed the mortality rate in early stages to 15% from 30%. The patients also reported no adverse side effects from Avigan.
- see the government announcement (in French)
- get more from Voice Chronicle