Boehringer Ingelheim has agreed to pay $650 million to wrap up thousands of lawsuits claiming Pradaxa, an anticoagulant, caused serious--even fatal--bleeding in some patients. The deal comes just weeks after the FDA completed a safety review of the drug, concluding that it was as safe as the older drug it seeks to replace, warfarin.
The Germany-based drugmaker said it stands behind the clot-fighting drug's safety. The settlement deal will allow it to avoid the cost and effort of long-running litigation, Boehringer said in a statement. The company said it's seeking to resolve 4,000 claims with the $650 million deal.
Pradaxa, the first in a new group of anticoagulants aiming to take the place of warfarin, has racked up thousands of reports of serious side effects since its U.S. launch in 2010. Boehringer added a warning to its label in 2011, urging doctors to test patients' kidneys before starting Pradaxa therapy, because subprime function could lead to a buildup of the drug in the bloodstream. But the company--and now the FDA--have assured doctors and patients that the drug is safe when used as directed.
 Those assurances came again today when the settlement was announced. "Time and again the benefits and safety of Pradaxa have been confirmed in many clinical trials and in real world data analyses. This settlement does not change the facts about Pradaxa or its importance to patients," General Counsel Andreas Neumann said in a statement. "From the time Pradaxa launched, Boehringer Ingelheim properly advised healthcare professionals and patients about its benefits and safety, working closely with ... regulators to ensure healthcare professionals and patients had the information they needed."
Those assurances came again today when the settlement was announced. "Time and again the benefits and safety of Pradaxa have been confirmed in many clinical trials and in real world data analyses. This settlement does not change the facts about Pradaxa or its importance to patients," General Counsel Andreas Neumann said in a statement. "From the time Pradaxa launched, Boehringer Ingelheim properly advised healthcare professionals and patients about its benefits and safety, working closely with ... regulators to ensure healthcare professionals and patients had the information they needed."
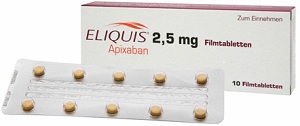
 The ongoing problem for Pradaxa, however, isn't competition with warfarin. It's the other new anticoagulants: Xarelto, from Johnson & Johnson ($JNJ) and Bayer; and Eliquis, from Pfizer ($PFE) and Bristol-Myers Squibb ($BMS). Study data and side-effects reports have suggested that Eliquis is the safer option, but it lags the other two, having been approved just last fall. Xarelto has successfully racked up a series of indications, and now boasts a bigger share of new prescriptions in the class.
The ongoing problem for Pradaxa, however, isn't competition with warfarin. It's the other new anticoagulants: Xarelto, from Johnson & Johnson ($JNJ) and Bayer; and Eliquis, from Pfizer ($PFE) and Bristol-Myers Squibb ($BMS). Study data and side-effects reports have suggested that Eliquis is the safer option, but it lags the other two, having been approved just last fall. Xarelto has successfully racked up a series of indications, and now boasts a bigger share of new prescriptions in the class.
Whether Pradaxa can bounce back from the bad publicity of lawsuits and safety reviews remains to be seen. But the drug could gain a major boost if its work toward a quick-acting antidote bears fruit. One of the key criticisms of the drug is that, unlike warfarin, it lacks an easy fix if and when bleeding starts. Warfarin's effects can be reversed with vitamin K; Boehringer--and, incidentally, its rivals--is making progress on developing a solution.
- read the Boehringer statement