 |
| The BEVS platform technology--courtesy of Protein Sciences |
A little more than a month after taking over vacated manufacturing space in New York from Pfizer ($PFE), Protein Sciences won regulatory approval from the FDA for a new type of flu vaccine.
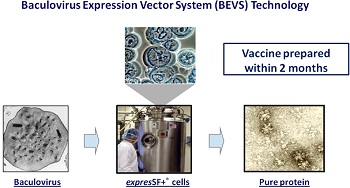
Unlike most flu vaccines, which are grown in chicken eggs, Flublok is made in insect cells. Consisting of a single protein from the virus--hemagglutinin--the vaccine can be ready weeks earlier than a traditional flu vaccine in the event of a pandemic. Manon Cox, the CEO of Protein Sciences, told The New York Times that Protein Sciences could prepare about 150,000 doses to distribute later this flu season.
Flublok relies on genetic code, which is available well before information on the seed strain, Protein Sciences Executive Chairman Daniel Adams told FierceVaccines. This means Protein Sciences, in theory, can get to manufacturing sooner than other companies.
"It's just inherently faster since you don't have to use a live flu virus," Adams said. "You don't have to use a live flu virus. You don't have to worry about safety. There's no safety issue with the genetic code."
Protein Sciences does not use actual flu virus to produce Flublok, thereby eliminating the risk of patients developing the flu upon receiving the shot.
Similarly, Novartis ($NVS) received approval in November for Flucelvax, a flu vaccine grown in mammal cells rather than eggs. Cell-culture technology also limits the time needed to manufacture large quantities of vaccine.
In December, Protein Sciences secured a lease on two buildings at Pfizer's Pearl River, NY, site to manufacture the vaccine. New York state officials showed their enthusiasm for the Meriden, CT-based company's move by providing Protein Sciences with $2 million in tax credits. The biotech hopes to employ 50 people in New York at first to meet an expected high demand for the recombinant vaccine.
- see the release
- get more from The New York Times (sub. req.)
Special Report: Top 10 selling flu vaccines of 2012