 |
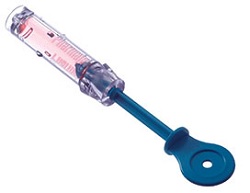
| A component of PharmaJet's Stratis needle-free jet injection system for vaccines--Courtesy of PharmaJet |
Colorado-based PharmaJet welcomed a nod from the FDA as the regulator approved the first needle-free delivery system for the inactivated flu vaccine. The jet injector has been shown to successfully provide vaccination without a needle jab, which could ultimately help improve vaccination rates.
The Stratis injection system is a reusable injector with a single-dose disposable cartridge that showed to be as effective as a needle and syringe in a prior study. In that study, the needle-free system delivered the Afluria three-strain vaccine using its fluid stream, which penetrates the skin in one-tenth of a second, according to the company.
The company claims that the injector eliminates needlestick injuries, needle reuse and possible cross-contamination.
"The PharmaJet injection technology is an especially important innovation for the millions of individuals who suffer from fear of needles and who consequently forgo their annual flu vaccination," PharmaJet CEO Ron Lowy said in a statement. "We believe this is a significant step forward in the effort to improve public health through broader immunization coverage, as well as improved safety of caregivers."
And on the safety side of things, a National Institute for Occupational Safety and Health study demonstrated that the incidence of needlestick injury is more than three out of every 100,000 vaccinations administered.
PharmaJet partnered with bioCSL, the maker of Afluria, to reach the approval.
- here's the release