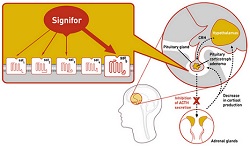
 Drugmakers are seeing more market potential in orphan drugs, and drug regulators are rewarding them with approvals. The FDA has for the second time this year approved an orphan drug for the rare Cushing's syndrome, this one from Novartis ($NVS). On Friday the agency gave approval to the Swiss company's Signifor, which is actually for Cushing's disease, a rare form of Cushing's syndrome.
Drugmakers are seeing more market potential in orphan drugs, and drug regulators are rewarding them with approvals. The FDA has for the second time this year approved an orphan drug for the rare Cushing's syndrome, this one from Novartis ($NVS). On Friday the agency gave approval to the Swiss company's Signifor, which is actually for Cushing's disease, a rare form of Cushing's syndrome.
The drug is approved for use when surgery to remove a tumor is not an option or has failed to provide relief. Cushing's disease is an endocrine disorder caused by excessive cortisol, which regulates metabolism and cardiovascular function and helps the body respond to stress. It affects only about one to two people in a million, mostly adults 20 to 50 years old and most often women. While rare, it is a difficult condition that can be fatal. Some of its symptoms include obesity, fatigue, high blood pressure and risk of infections.
The European Commission approved Signifor in April, and applications are pending before regulators in other countries, Novartis said. A spokeswoman for Novartis said the company would not comment on market potential for the drug.
Drugmakers small and large are developing for rare diseases, a field that targets unmet medical needs and comes with extended exclusivity periods and lower costs of clinical development. In fact, in February the agency approved Corcept Therapeutics' ($CORT) drug Korlym (mifepristone), which is the first FDA-approved treatment for Cushing's syndrome. Korlym, which was granted orphan status in 2007, is expected to benefit about 5,000 patients in the U.S., and its approval was based largely on a clinical trial involving just 50 patients.
An FDA panel of outside experts in November had unanimously recommended Signifor, but it comes with some serious side effects. Those include an increased risk for liver damage and diabetes. The FDA has required Novartis to do three postmarketing studies for Signifor: A clinical trial to assess high blood sugar management; one to create a long-term prospective registry of patients with Cushing's disease treated with Signifor; and one to track reports of serious hyperglycemia, acute liver injury and adrenal insufficiency.
- here's the FDA release
- and the Novartis release
- more from Pharmafile
Related Articles:
FDA experts give Novartis' Cushing's drug unanimous endorsement
Corcept skyrockets on FDA nod for rare disease drug
FDA review endorses efficacy of Novartis' new drug for Cushing's disease