 |
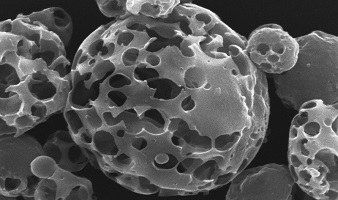
| Novartis' Pulmosphere particles allow drug delivery via a dry powder rather than a nebulized liquid.--Courtesy of Novartis |
The FDA approved Novartis' ($NVS) cystic fibrosis treatment that fights bacterial infection via a powder solution inhaled from a handheld device, the first of its kind that is administered in this way.
The mode of delivery for the pharma giant's TOBI Podhaler allows for a much more manageable treatment regimen to curb the growth of bacteria in the thick buildup of mucus associated with cystic fibrosis. The bacterium Pseudomonas aeruginosa in particular has been known to cause lung infections, and previous treatments included the use of a larger nebulizer machine to vaporize liquid drugs.
The TOBI Podhaler is the first handheld inhaler able to deliver tobramycin, an antibiotic used to treat the infection. Novartis' Pulmosphere allows for the creation of hollow, porous particles of tobramycin, delivering the drug in powder form, according to the company.
Besides the convenience of a handheld device, the company also touts the product's shortened time commitment, cutting down on administration of the treatment by 70%. CF already places a heavy burden on patients with an average daily treatment time of 108 minutes.
But Novartis still trails behind Vertex ($VRTX) in the novel treatment of cystic fibrosis--while drugs like tobramycin treat the symptoms and infections caused by the disease, Vertex's Kalydeco is the first to address underlying genetic abnormalities.
- here's the FDA release
- read more from FierceBiotech
Related Articles:
Novartis, Bayer, Roche score regulatory nods on 3 continents
Novartis snags FDA approval for antibiotic in CF patients
Vertex plots a race through PhIII for 'breakthrough' combo CF therapy