 |
| The EMA will launch a review of contraceptive safety--courtesy of Planned Parenthood |
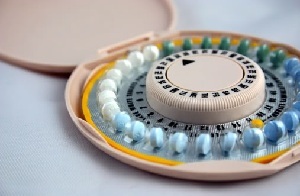
The European Medicines Agency is launching its own review of contraceptive safety. At the request of France, which zeroed in on the risk of blood clots with third- and fourth-generation birth control pills, EMA says it will also sift the data.
The agency's Pharmacovigilance Risk Assessment Committee will review the newer oral contraceptives, EMA said in a release, saying that this is the first time that a member state has asked the agency to deliver a recommendation under its new pharmacovigilance legislation. Previously, EMA had said it saw no additional clotting risk with the newer pills.
France's ANSM health regulator launched a probe early this year, saying that doctors may be over-prescribing third- and fourth-generation birth-control pills. French officials said the newer pills should only be prescribed by specialists. The government plans to tighten up reimbursement for the newer pills because of its safety concerns.
The French moves follow FDA's long-term review of some lower-dose contraceptives. After new studies suggested a higher risk of serious blood clots with birth-control pills containing drospirenone--the fourth-generation pills--the agency in 2011 asked an advisory committee to weigh the data. The committee backed the pills in a 15-11 vote but suggested a labeling update. Then, in April, the FDA added data on the pills' clotting risks to their official labels. Bayer maintains that its own research hasn't turned up any additional risk with its newer pills when compared with older contraceptives.
The EMA committee meets next week, and more information about the review will be available afterward, the agency said.
- read the EMA release