GlaxoSmithKline's ($GSK) candidate and Merck/NewLink's jab will be the first of several experimental Ebola vaccines to undergo large-scale clinical trials. On Friday, Glaxo shipped an initial batch of 300 vials of the vaccine, ChAd3, to Liberia, one of the main countries affected by Ebola.
The Phase III trial, led by the NIH, is expected to involve up to 30,000 people, one third of whom will receive GSK's candidate. A second group will receive a placebo, and the third group will receive the vaccine developed by Merck ($MRK) and NewLink ($NLNK), reported CNBC.
Placebo-controlled studies are "considered the gold standard" to assess safety and efficacy, Dr. Anthony Fauci, director of the NIH's National Institute of Allergies and Infectious Diseases (NIAID) told reporters in a conference call, as reported by CNBC. The NIAID is collaborating with Glaxo on the vaccine.
Investigators will assess whether the immune response seen in Phase I trials actually confers meaningful protection against Ebola, GSK said in a release.
"The initial phase I data we have seen are encouraging and give us confidence to progress to the next phases of clinical testing which will involve the vaccination of thousands of volunteers, including frontline healthcare workers," said Dr. Moncef Slaoui, chairman of global vaccines at GSK. "If the candidate vaccine is able to protect these people, as we hope it will, it could significantly contribute to efforts to bring this epidemic under control and prevent future outbreaks."
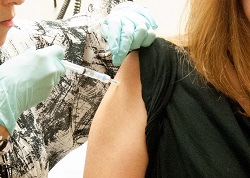 |
| A woman receives a dose of GSK's ChAd3 during trials at the NIH Clinical Center.--Courtesy of NIAID |
The candidate uses a chimpanzee cold virus as a vector to carry segments of genetic material from the Zaire strain of the ebolavirus. It is already being tested in Phase I trials in the United States, the U.K., Switzerland and Mali. The initial data show that the vaccine has an acceptable safety profile. Further results will be published in the coming months.
Glaxo is working with the WHO and the CDC to design and potentially support trials in Sierra Leone and Guinea in the months ahead. According to the release, it also plans to begin large Phase II trials in unaffected West African countries.
In addition to Merck and NewLink, Johnson & Johnson ($JNJ) and Bavarian Nordic are collaborating on another candidate.
"It is important to remember that this vaccine is still in development and any potential future use in mass vaccination campaigns will depend on whether WHO, regulators and other stakeholders are satisfied that the vaccine candidate provides protection against Ebola without causing significant side effects and how quickly large quantities of vaccine can be made," Slaoui said.
- get the release
- here's FierceBiotech's take
- see more from CNBC
Special Report: 10 drugs that could stop Ebola