 |
| Cerulean's tumor-penetrating nanotech--Courtesy of Cerulean |
A Cerulean Pharma ($CERU) announced last week that the first patient has received its Phase I/IIa cancer candidate, CRLX301, delivered using its Dynamic Tumor Targeting Platform.
The candidate's active ingredient is docetaxel, used in Sanofi's ($SNY) Taxotere to treat breast cancer, non-small cell lung cancer and prostate cancer, as well as Sun Pharma's generic cancer medication Docefrez. A member of FierceBiotech's Fierce 15 class of 2011, Cerulean says preclinical data show that its nanoparticle-drug conjugate (NDC) version of docetaxel appears safer and more efficacious than those older medications and could have new therapeutic benefits in the treatment of multidrug-resistant tumor models.
"CRLX301 was superior to docetaxel in seven of seven animal models, with a statistically significant survival benefit in five of those preclinical models," said Cerulean Executive Chairman Dr. Paul Friedman in a statement. "We also saw preclinical tolerability that is consistent with our expectations of the platform--specifically, improved tolerability relative to published data with docetaxel. We look forward to learning if these preclinical results will translate in the clinic."
On the company website, Cerulean says its RNA delivery program offers various advantages including the protection of RNA payloads during administration, sustained release, and the delivery of the RNA directly into tumor tissue.
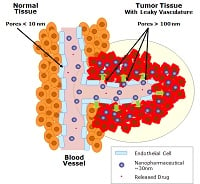
MIT's Robert Langer, who serves as one of the company's scientific advisers, explained the company's drug delivery technology to FierceDrugDelivery in 2013, saying that its main platform targets tumors through large pores in the blood vessels, keeping healthy cells intact because they are too large to pass through their smaller pores.
Docetaxel belongs to a class of drugs called taxanes that target cancer cells' microtubules and are of great interest to the company. Chief Medical Officer Dr. Edward Garmey said in a statement that "during the course of the CRLX301 clinical development program, we will carefully assess the investigational spaces in which currently approved taxanes can be improved through the targeted delivery of the cytotoxic payload to tumor cells and the relative sparing of healthy cells. We also are interested in those settings where taxanes haven't been approved but where our NDC technology might facilitate their use, either alone or in combination."
The company's lead candidate, CRLX101, failed a Phase IIb study in 2013, missing an ambitious main endpoint of overall survival in patients with non-small cell lung cancer, though the company still believes that it has a lot of potential.
Cerulean's stock price has fallen by about 80 cents to around $6.20 since its IPO at $7 on the Nasdaq in April 2014.
- read the release
- here's the candidate's website
Special Report: FierceBiotech's 2011 Fierce 15 - Cerulean Pharma- 2011 Fierce 15