The report outlines contributions by Purdue Pharma, Johnson & Johnson's Janssen, Depomed, Insys Therapeutics and Mylan to 14 patient organizations and affiliated individuals between 2012 and 2017. With pharma backing, the groups "amplified messages favorable to increased opioid use," according to the report.
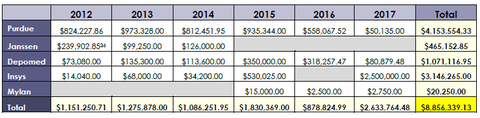
Purdue Pharma made more than $4 million in payments to the groups, according to the senator's report, followed up by Insys' $3-million-plus in contributions. Depomed contributed more than $1 million during the period, while Janssen paid $465,000 to the groups. At just over $20,000 in payments, Mylan shelled out only a small fraction—.2%—of the group's total. The report comes just days after Purdue Pharma said it would stop marketing OxyContin and other opioid pain meds to physicians. With that change, the company plans to lay off 200-plus sales reps, or more than half its staff.
Additionally, doctors affiliated with the groups have accepted more than $1.6 million in payments from the companies since 2013, according the report.
The advocacy groups downplayed opioid addiction risks, promoted opioids to treat chronic pain, lobbied to change laws that sought to curb opioid use and fought to reduce accountability for individuals who overprescribed or misbranded the meds, according to the report.
Mylan hit back, saying its contributions were only a tiny fraction of the total. Depomed said it has promoted its opioid drugs responsibly. Insys responded that its contributions in 2017 were "patient focused."
Citing the City of Chicago's lawsuit against multiple opioid drugmakers, the report shows that in one case, the American Academy of Pain Medicine and the American Pain Society released a 1997 statement that "endorsed opioids to treat chronic pain and claimed that the risk that patients would become addicted to opioids was low." A Purdue-backed speaker who is now the company's VP of Health Policy co-authored the statement, according to the city's complaint.
When the CDC came out with opioid prescribing guidelines in 2016, many of the groups sought to "criticize and undermine" the new guidance, according to McCaskill's report.
RELATED: Chicago makes second run at Allergan, J&J, Purdue and others with painkiller lawsuit
The "ostensibly neutral advocacy organizations have often supported industry interests at the expense of their own constituencies," the report states. The U.S. Pain Foundation received nearly $3 million in contributions from the drugmakers over the period, Sen. McCaskill's team found. The Academy of Integrative Pain Management and American Academy of Pain Medicine each received more than $1 million.
In a statement, U.S. Pain Foundation president Paul Gileno said that the money "does not influence our values" and that the contributions don't steer the group toward any specific treatment options.
"We support a balanced, multidisciplinary approach to pain care," he added.
Including Mylan in the executive summary is "another example of Washington putting politics over people," a Mylan spokeswoman said in a response today. Mylan, as she noted, contributed only $20,250, compared to millions for some of the other drugmakers.
She wrote that "lumping Mylan in with this group of companies is not only highly irresponsible, but also highlights more of a political agenda rather than finding real solution to the opioids crisis."
RELATED: CDC guidelines call for drastic cuts in opioid painkiller use
Depomed said its contributions "covered corporate advertising, conference booth fees, sponsoring training certifications and membership fees." Further, the drugmaker said it believes it has "acted responsibly with respect to the marketing and advertising of the Lazanda and Nucynta franchises" and that it has a strong compliance program in place to oversee the activities.
Insys, which has had a number of ex-employees charged for the the company's earlier marketing practices, said it has been cooperating with the probe since July 2016.
"We believe that our 2017 charitable contributions, which have become the subject of media attention, are patient focused," the company's statement said. "With regard to the U.S. Pain Foundation specifically, our donation was directed to a disease-state fund for cancer patients with breakthrough pain, which many medical experts and healthcare providers believe is significantly undertreated in the U.S."
The company added that "existing laws and regulations ... permit these types of charitable contributions that benefit patients."
A Janssen spokesperson told FiercePharma the contributions "were made to support efforts to educate the public about the appropriate use of opioid pain medicines, and were transparently disclosed."
Purdue didn't immediately respond to a request for comment.
RELATED: As lawyers push to limit opioid production in legal deal, Sen. Sanders wants more from Congress
Sen. McCaskill's report comes as opioid drugmakers face more than 200 lawsuits from cities and counties over their marketing practices; lawyers in those cases met recently in Cleveland to talk about a global settlement, according to multiple reports. Judge Daniel Polster wrote in a recent court filing that the discussion was "productive." The next settlement conference is scheduled for March 6.
Separate from those cases, attorneys general from dozens of states have joined together to investigate the opioid industry's marketing.
Editor's note: This story was updated with statements from Janssen and the U.S. Pain Foundation.