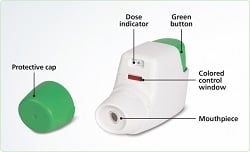
 |
| Tudorza Pressair inhaler--Courtesy of AstraZeneca |
AstraZeneca ($AZN) had to recall inhalers in Denmark last year because some of them may have been empty. Now it's doing the same in the U.S. where some of its Tudorza Pressair inhalers may be defective.
According to the most recent FDA Enforcement Report, the U.K. company is voluntarily recalling nearly 150,000 Tudorza Pressair inhalers manufactured in Spain because the delivery system may be improperly set and may not provide the full 60 doses of aclidinium bromide inhalation powder they are designed to deliver.
AstraZeneca bought both the Tudorza Pressair and Daliresp respiratory meds last year from what is now Allergan ($AGN) for $600 million and royalties. AstraZeneca has bought a number of respiratory products in the last few years to boost its place in that category as it faced losing patent protection on blockbuster asthma med Symbicort. In 2014 it spent more than $2.2 billion for the respiratory meds of Spain's Almirall.
The company in August announced it is retrieving one lot of its Bricanyl Turbuhaler in Denmark after discovering they may contain no powder. Other drugmakers have had inhaler issues as well. In December, GlaxoSmithKline ($GSK) recalled nearly 130,000 Ventolin HFA inhalers with propellant issues. Months earlier, Boehringer Ingelheim recalled about 360,000 Combivent Respimat inhalers, used to treat chronic obstructive pulmonary disease, because the delivery system was defective and may provide insufficient spray, or none at all. Both of those recalls were in the U.S.
- access the report here