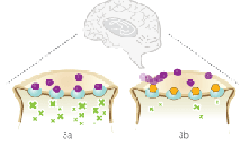
 |
| Buprenorphine (orange) binds to the mu opioid receptors in the brain to block opioids from attaching to them. Titan hopes to commercialize the implant to deliver the drug, which is currently available in sublingual tablet and oral formulations.--Courtesy of Titan Pharmaceuticals |
The first long-acting subdermal implant to treat opioid addiction moved a step closer to FDA approval when the agency's Psychopharmacologic Drugs Advisory Committee recommended approval of the drug-device combination product by a vote 12-5.
Princeton, NJ's Braeburn Pharmaceuticals and development partner Titan Pharmaceuticals hope the FDA follows the recommendation in favor of Probuphine for the delivery of buprenorphine, something it is not required to do, as evidenced by the 2013 rejection of the implant in spite of a positive recommendation.
The FDA once again raised questions about the application in a document submitted to committee members, reports MedPage Today.
At issue was the response rate and analysis of missing clinical trial data. FDA reviewers concluded that at 69% (compared to 64% for sublingual buprenorphine), Probuphine's response was non-inferior to the standard of care, while Braeburn submitted a superiority claim.
"Under some sets of assumptions, one might question whether enough of the effect size has been maintained to conclude efficacy of Probuphine," the FDA wrote, adding, "Moreover, because Probuphine ensures compliance, one would expect a clearer demonstration of superiority over sublingual buprenorphine than was demonstrated in this trial." And the agency compared Probuphine to a drug delivering contraceptive that isn't on the market any longer because of difficulty inserting and removing the implant.
The FDA raised concerns about the trial data and the installation and removal of the Probuphine implant in 2013 as well. Still, non-inferiority was enough to convince most of the panelists, though they did express some reservations.
"The sponsor's analysis was incomplete, but the FDA's analysis was thorough," said panelist of the NIH's National Heart, Lung, and Blood Institute, according to MedPage Today. "They used conservative assumptions that led to [the drug] still being able to pass a small non-inferiority margin, so I was convinced by that."
The vote in favor of approval came in the form of a single question asking whether the efficacy, safety and risk benefit profile of Probuphine was good enough to merit commercialization.
"Probuphine has the potential to be the first marketed product to provide maintenance treatment of opioid addiction continuously for six months following a single procedure. As a subdermal implant, Probuphine could increase patient compliance, decrease the risk of diversion and improve patients' quality of life. We look forward to the Agency completing its review of the NDA," said Titan Pharmaceuticals President and CEO Sunil Bhonsle in a statement.
The stakes are higher this time around, given the increased attention on opioid abuse, a growing problem that's being discussed by the presidential contenders, and has been implicated in the startling rise of the death among white Americans without a college degree.
- read the release
- here's MedPage Today's take | the publication's review of the FDA literature
- here's more about the meeting