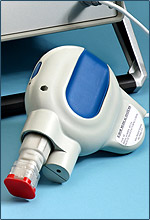
 |
| The TriGrid delivery system--Courtesy of Ichor Medical Systems |
San Diego biotech Ichor Medical Systems stands to earn up to $85 million, plus royalties, if its new partnership with Johnson & Johnson's ($JNJ) Janssen hits the right notes. The companies announced plans this week to develop DNA vaccines for hepatitis B using Ichor's TriGrid electroporation technology for clinical administration.
The company's TriGrid device uses brief electrical pulses to alter cell membranes and allow DNA to enter cells. According to a release, the tech has been shown to "significantly enhance" immune responses to delivered vaccines compared to conventional injection.
DNA vaccines, though emerging, have seen limited efficacy in traditional injection. The vaccines generate antigen-specific antibodies and T cells that are key for a long-term resolution of hepatitis B, Ichor said in a statement. Through the partnership, the startup will receive an upfront payment, R&D support and development and sales milestone payments, plus potential royalties. Janssen will assume commercialization costs associated with the program plus manufacturing costs and distribution of the TriGrid system.
"Janssen's decision to couple our clinically validated TriGrid electroporation technology with their DNA vaccine for HBV is extraordinarily exciting for Ichor," said Ichor CEO Robert Bernard. "Our scientists and engineers look forward to this opportunity to collaborate with Janssen in the development of immunotherapies for patients suffering from chronic hepatitis B infection."
More than 2 billion people alive have been infected with hepatitis B, and more than 240 million people worldwide remain infected, the release stated.
With its technology, Ichor is no stranger to joining with high-profile partners for vaccines R&D. In February 2014, it partnered with Pfizer ($PFE) to use TriGrid in conjunction with the pharma's preclinical cancer vaccine-based immunotherapies. It also partnered with the U.S. government's Defense Advanced Research Projects Agency to test TriGrid in delivering antibodies for passive immunoprophylaxis.
- here's the release